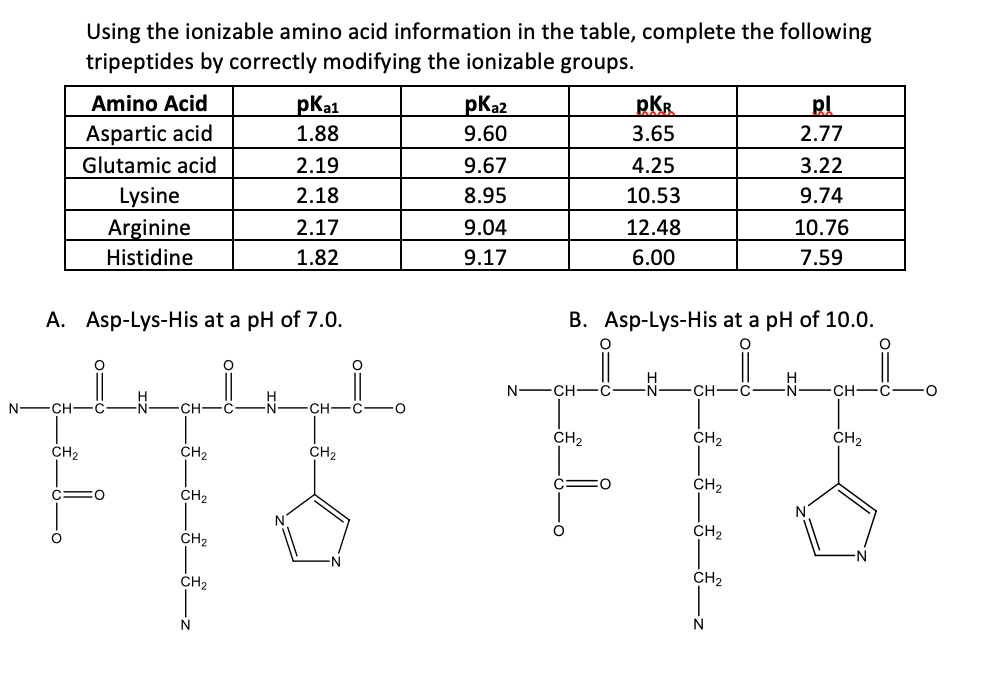
The structure of aspartic acid is given below: NH (HOOC CH-CH2COOH) (A) The pKa,,pka, and pKaz of (A) respectively, are 1.88, 3.65 and 9.60. pka, and pka2 corresponds to the ionization of

Titration of Amino Acids | pH, pKa1 and pKa2 | Amino Acids (Part 4). | By Medicosis Perfectionalis | Facebook
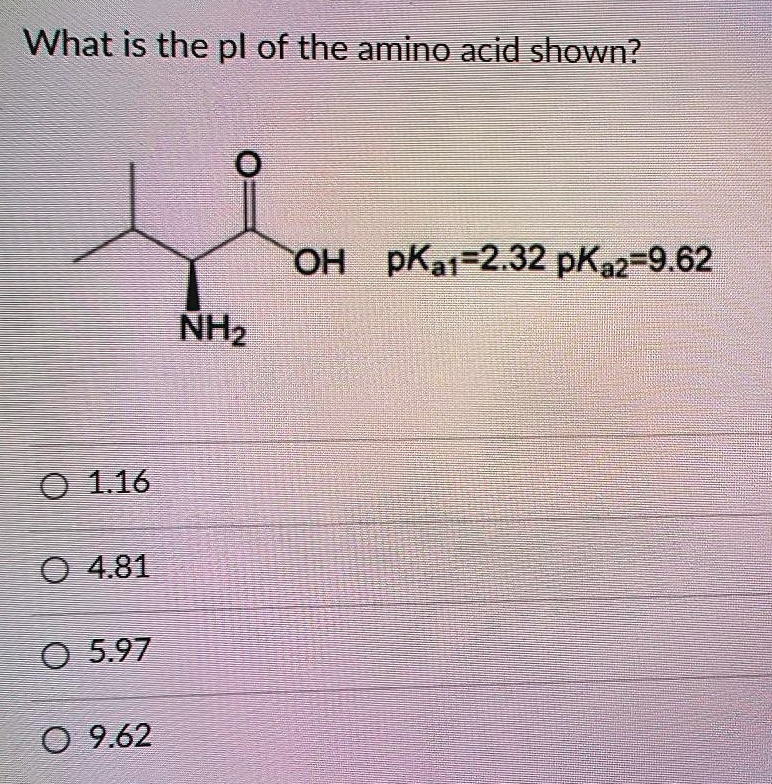
SOLVED: What is the pKa of the amino acid shown? OH pKa1 = 2.32, pKa2 = 9.62 NH2 1.16 4.81 5.97 9.62
Lecture 16: Polyprotic Acids We should be pretty comfortable dealing with monoprotic acids like: HCl HNO3 HClO4 CH3COOH +HN